Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM

1. Terms and Definitions
Silicon halides (mainly monomethyltrichlorosilane, silicon tetrachloride or trichlorosilane) are hydrolyzed and burned in a hydrogen-oxygen flame at 1000°C or higher to form primary silica particles, which then collide with each other to form secondary particles and long chains, generating ultrafine white powder with surface hydroxyl groups and adsorbed water, which is fumed silica (abbreviated as fumed silica).
The specific production process is called chemical vapor deposition (CAV), also known as thermal decomposition, dry process, or combustion process. The raw materials are generally silicon tetrachloride, oxygen (or air), and hydrogen, which react at high temperatures according to the reaction formula: SiCl₄ + 2H₂ + O₂ → SiO₂ + 4HCl. The air and hydrogen are pressurized, separated, cooled and dehydrated, dried on silica gel, and filtered for dust removal before being fed into a synthetic hydrolysis furnace. The silicon tetrachloride raw material is then distilled in a distillation tower and heated in an evaporator for evaporation. The dried, filtered air is then fed into the synthetic hydrolysis furnace. After silicon tetrachloride is gasified at high temperature (flame temperature 1000~1800℃), it undergoes gas-phase hydrolysis at a high temperature of about 1800℃ with a certain amount of hydrogen and oxygen (or air). The gaseous silicon particles generated at this time are extremely fine and form aerosols with the gas, which are difficult to capture. Therefore, they are first aggregated into larger particles in an aggregator, then collected by a cyclone separator, and then sent to a deacidification furnace. The gaseous silicon is purged with nitrogen-containing air to a pH value of 4~6 to obtain the finished product.
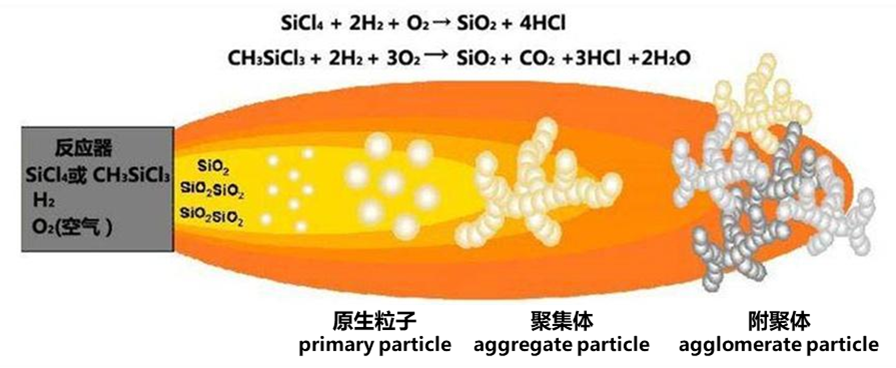
The primary particle size of fumed silica is 7-14 nm, and the nitrogen adsorption specific surface area (NSA) is 70-400 m²/g. After being fully dispersed, the particle size is small, reaching a nanometer state. It has high surface activity and has excellent reinforcement, thickening, thixotropy, matting, anti-settling, anti-sagging, UV resistance and sterilization effects. It can effectively improve the application performance of coating products, for example: preventing the sedimentation and stratification of pigments and fillers, improving the weather resistance and scratch resistance of coatings, and improving the bonding strength between coatings and substrates.
2.Relevant Standards
GB/T 20020-2013 “Fumed Silica” (soon to be replaced by GB/T 20020-2025)
Q/(GZ) GBS01-2002 “Fumed Silica”
T/FSI 490-2020 “Test Method for Surface Silicyl Content of Fumed Silica”
ISO 3262-20:2000(E) “Coatings and Additives — Description and General Methods — Part 20: Fumed Silica”
GB/T 20020-2013 classifies fumed silica into hydrophilic Type A and hydrophobic Type B. Type A fumed silica is not coated with organic matter, while Type B is made from Type A products that have been surface-modified with organic matter. The standard also stipulates that the product name of fumed silica consists of the type code (A or B) followed by a typical NSA value. For example, “A300” indicates a Type A fumed silica with a typical NSA value of 300 m²/g. In addition to the NSA, the standard also specifies technical indicators and related measurement methods for different types of fumed silica, such as loss on ignition, various impurity contents, suspension pH, and tap density.
GB/T 20020-2025 was recently released (August 1) and will be officially implemented on February 1 next year, replacing the current GB/T 20020-2013.
3.Application of fumed silica in coatings
Fumed silica particles are small, have a large surface area, and are rich in silanol groups. This large surface area allows for significant interaction with other ingredients in coating formulations. Once dispersed in a coating, they form a three-dimensional network that impedes liquid flow, increasing viscosity and creating thixotropy, preventing the coating from settling during storage. Shear forces disrupt this branched network, causing a decrease in viscosity (shear thinning). After coating, the fumed silica particles reform their network, and the coating’s viscosity returns to a high level (thixotropy), ensuring leveling without sagging and ensuring the coating remains stable at rest during the curing phase.
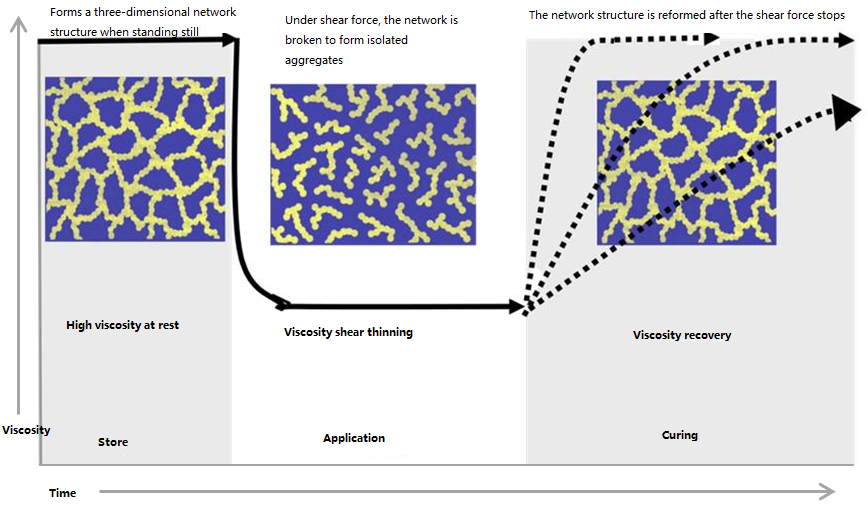
Fumed dioxide can also modify the surface properties of coatings and enhance adhesion by interacting with the substrate surface. The high surface area and surface chemistry of fumed dioxide promote adhesion between the coating and the substrate, thereby improving adhesion and coating integrity. For example, as a rheology modifier, fumed dioxide is well-suited for high-build coatings (such as epoxy zinc-rich primers), ensuring good flow under certain application shear forces while maintaining a consistent film thickness. During application, rapid solvent evaporation at the edges of the coating creates uneven surface tension, which can easily cause the coating to migrate toward the edges. Fumed dioxide effectively prevents this migration, creating a thick edge, and also prevents sagging during the curing process, ensuring a uniform coating.
Fumed dioxide is commonly used in paints and coatings as a rheology modifier, anti-settling agent and dispersant.
1). Rheology control additives
Fumed silica has numerous hydroxyl groups on its surface, which form hydrogen bonds between its aggregates. When fully dispersed in a coating, this creates a silica network structure.
During application, rapid solvent evaporation at the edges of the coating leads to uneven surface tension, which can easily cause the coating to migrate toward the edges. The silica network effectively prevents this migration and the formation of edges. It also prevents sagging during the curing process, ensuring a uniform coating.
On the other hand, fumed silica, due to its ability to form hydrogen bonds, increases the medium- and low-shear viscosity of the system, acting as a thickener and producing a thixotropic effect. For this reason, fumed silica is widely used in oil-based systems.
Furthermore, fumed silica has a large specific surface area and high surface energy, making it prone to agglomeration. Therefore, proper dispersion is essential during application to achieve optimal results. Insufficient dispersion prevents the formation of a complete three-dimensional network structure, while overdispersion completely destroys the network between silica particles, making it difficult to restore even after prolonged shearing.
During the coating production process, the surface treatment of fumed silica, the method of addition, and the choice of dispersing equipment all affect its dispersion in the coating. Generally, it is best to first disperse the fumed silica into a pre-slurry before adding the powder for complete dispersion.

2). Anti-settling agent
Fumed silica has a small particle size and a large surface area, and its surface contains silanol groups. These silanol groups can interact with neighboring fumed silica particles, forming hydrogen bonds.
This hydrogen bonding creates a thixotropic structure. The suspending effect of fumed silica arises from the network structure it forms when dispersed in a solution. This network prevents particles from agglomerating and phase separation. Furthermore, the increased viscosity of the system helps prevent the movement of components within the system.
As an ideal anti-settling agent, fumed silica is very effective in preventing pigment precipitation in coating systems. In particular, for pigment paste systems, an appropriate addition significantly improves pigment stability and reduces the amount of wetting and dispersing agent, improving the pigment’s applicability and minimizing its impact on the coating system. The hydrogen bonding creates a thixotropic structure.
Fusibly, the anti-settling effect of fumed silica is highly beneficial for paint storage. Certain pigments, such as metal powders and flakes, are particularly prone to settling and cannot be fully suspended. Fumed silica ensures their dispersion without settling. Based on the total formulation, the silica dosage ranges from 0.4% to 0.8%, but in special cases, such as zinc-rich paints, it may need to be increased to 2%. In UV coatings, the addition level can be adjusted (0.5% to 2.5%) to optimize viscosity thixotropy, abrasion resistance, and matting effects.
3). Dispersing aid
Fumed silica dispersed in paint strengthens the bond between the paint matrix and inorganic fillers, improving mechanical properties such as hardness, scratch resistance, and durability. Adding fumed silica to facade paint effectively prevents sagging, improves the smoothness of the construction surface, and significantly enhances the scrub resistance and aging time of exterior wall paints. It also significantly increases the bond strength between the coating film and the wall, significantly increases the coating film hardness, and improves the surface’s self-cleaning ability.

4). Matting performance
Fumed silica, due to its tiny particle size and high specific surface area, forms a three-dimensional network structure, resulting in significant light scattering capabilities. Its refractive index, 1.46, is similar to that of most resins used in the coatings industry. This results in excellent optical properties in clear varnishes, making it a preferred matting agent for high-end coatings.
The key parameters influencing the performance of fumed silica matting agents include pore volume (porosity), average particle size and particle size distribution, and surface treatment. For a given fumed silica matting agent, its matting efficiency increases with increasing pore volume. Average particle size and its distribution play a significant role in reducing coating gloss. For a given coating and film thickness, matting effectiveness is best achieved when the matting agent particle size corresponds to the coating dry film thickness. The narrower the particle size distribution band, the higher the matting efficiency. Fumed silica effectively reduces coating gloss while maintaining transparency. Its matting efficiency surpasses that of traditional fillers such as calcium carbonate.
5). Other application features
(1) After being surface-treated with methyl methacrylate silane, fumed silica can be added to polyurethane coatings to achieve friction resistance. 5% to 15% of fumed silica is firmly embedded in the coating film, and the aggregated particles are partially exposed on the surface of the coating after film formation, forming micro-roughness. This microstructure makes the coating surface non-slip and non-adhesive, and the friction coefficient is greatly improved, which can be increased by 10% to 35%. At the same time, the rheological properties of the coating and the optical properties of the dry film are not negatively affected.
(2) It can also improve the weather resistance and scratch resistance of the coating and improve the bonding strength between the coating and the substrate.
(3) It has extremely strong ultraviolet absorption and infrared light reflection properties. Adding it to the coating can improve the anti-aging properties of the coating.
(4) Fumed silica has high surface activity. After modification, its surface charge can be adjusted. Pigments or fillers may aggregate or agglomerate due to their activity. Fumed silica can neutralize these charges, prevent the agglomeration of pigments or fillers, and improve their dispersibility and storage properties. In powder coatings, silica can be adsorbed on the surface of the powder coating to form a movable layer, producing a “balling” effect, preventing the powder coating from absorbing moisture and agglomerating, and improving the fluidity of the powder coating.

4.Outlook and precautions for fumed silica for coatings
Manufacturing, both now and in the future, is trending toward functionalization and greening, leading to the emergence of environmentally friendly and functional coatings. Fumed silica manufacturing must also evolve toward functionalization and personalization. To maximize the performance of fumed silica, manufacturers should closely collaborate with coating users, fully considering key factors such as its dispersion and stability in the coating, and paying attention to the impact of the coating system during formulation design.
It is also important to note that dispersing or grinding equipment is crucial to fully realizing the excellent properties of fumed silica. Only when it is fully dispersed in the coating can its optimal rheological properties be achieved. Good dispersion depends on the applied shear force (disperser design, size, speed, and power) and the dispersion time. Insufficient shear force will not achieve optimal dispersion, even with extended dispersion time.