Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Polyurethane foam products are currently the most widely used and consumed polyurethane product, categorized into three main types: flexible, rigid, and semi-rigid. In 2020, my country’s polyurethane foam consumption reached approximately 4.67 million tons. Of this total, polyurethane flexible foam accounted for 2.06 million tons, or 44.1%, while soft polyurethane foam accounted for 2.61 million tons, or 55.9%. Flexible polyurethane foam (PU soft foam) is a flexible polyurethane foam with a certain degree of elasticity, having a molecular weight of 2,000 to 4,000 and a density of 16 to 192 kg/m³. It is the most consumed polyurethane product.
The one-step process flow of Flexible polyurethane foam is as follows:
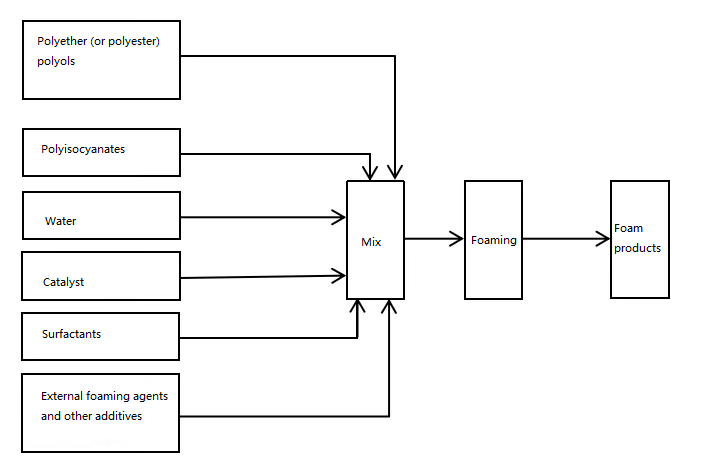
As shown in the diagram above, the PU foaming reaction is based on two main chemical components: polyether polyol and isocyanate. Other components, including water, trichlorofluoromethane, foam stabilizers, and catalysts, are added simultaneously. These materials mix and react instantly and intensely at high speed, forming foam. This process releases a large amount of heat. Foam plastic is a porous material with a large specific surface area. While heat can be dissipated from the edges of the foam, it is more difficult to remove heat from the center due to the foam’s excellent thermal insulation. During a normal reaction, the heat released raises the center of the foam block to a certain temperature, achieving the purpose of maturation.
Measurements show that the temperature can rise to 140-160°C within 2-6 hours of foaming, sometimes even higher, around 180°C. If the temperature continues to rise, it can cause core burning, smoking, and even spontaneous combustion. Furthermore, when polyurethane soft foam is exposed to sunlight for a long time, it will undergo auto-oxidation, leading to polymer degradation, discoloration, brittleness, and a decrease in physical properties, ultimately rendering it useless. Since the industrialization of polyurethane, core burning and aging have been a hot topic of research and concern within the polyurethane industry.
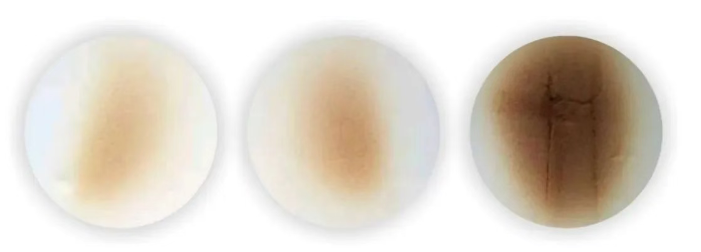
Antioxidants are key additives for polyurethane foam. Appropriate antioxidants prevent polyol decomposition, reduce byproduct production, lower the risk of core burn, and delay thermal oxidative aging during product use, thereby extending the product’s service life. Commonly used antioxidants for PU flexible foam are liquids and fall into three main categories: aromatic amines (such as 5057), hindered phenols (such as 1135), and phosphites (such as PDP). For applications with less stringent color requirements, antioxidants typically use a combination of aromatic amines and hindered phenols; for applications with more stringent color requirements, a combination of hindered phenols and phosphites is often used.
Furthermore, if the product is frequently exposed to sunlight, a certain amount of anti-UV additives, primarily consisting of light stabilizers and light shielding agents, is required to improve its lifespan and yellowing resistance. Light stabilizers primarily fall into two categories: UV absorbers and hindered amine light stabilizers. UV absorbers primarily include benzotriazoles, benzophenones, and triazines. These absorb harmful UV radiation and convert it into heat through intramolecular hydrogen bond transfer or cis-trans isomerization. Hindered amine light stabilizers are amines with two methyl steric groups attached to the two alpha carbon atoms of the amino group. These light stabilizers are converted into nitroxyl radicals upon photooxidation. These nitroxyl radicals are considered truly stable components that can capture free radicals, with the resulting products then reacting with peroxide radicals to regenerate nitroxyl radicals. Light shielding agents include pigments such as carbon black, zinc white, and titanium white, which are used as colorants.
Light shielding agents use their high dispersibility and hiding power to reflect harmful ultraviolet rays back and protect polymers.