Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Work Hours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM

1.What are surfactants?
A solute that can significantly reduce the surface tension of water after addition is called a surfactant, commonly known as a surfactant.
Surfactants are special organic compounds with an amphiphilic molecular structure: a hydrophilic group at one end and a hydrophobic group at the other. This property enables surfactants to form two interfacial (surface) adsorption functions in aqueous solutions, significantly reducing the surface tension of water. Surfactants are typically organic compounds containing hydrophilic polar groups and hydrophobic nonpolar carbon chains or rings. When the hydrophilic groups enter water, the hydrophobic groups attempt to leave the water and migrate toward the air, forming a directional alignment at the interface.
The surface concentration of surfactant is greater than the bulk concentration, and the work required to increase the unit area is less than that of pure water. The greater the non-polar component, the greater the surface activity.
2.Classification of surfactants
Surfactants are usually classified according to their chemical structure and are divided into two categories: ionic and non-ionic. Ionic surfactants can be further divided into cationic, anionic and amphoteric surfactants.
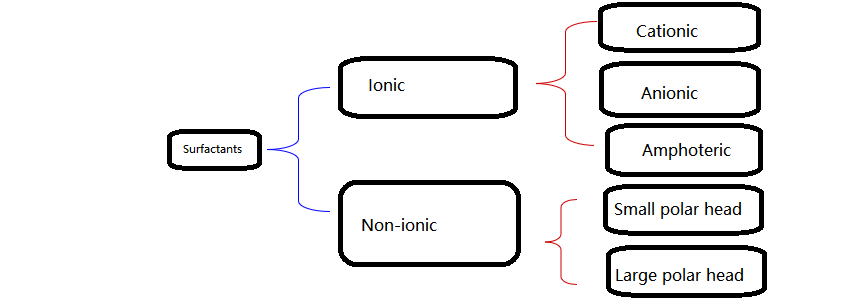
Note: Cationic and anionic surfactants cannot be mixed, otherwise precipitation may occur and the activity will be lost.
3.Common surfactant types
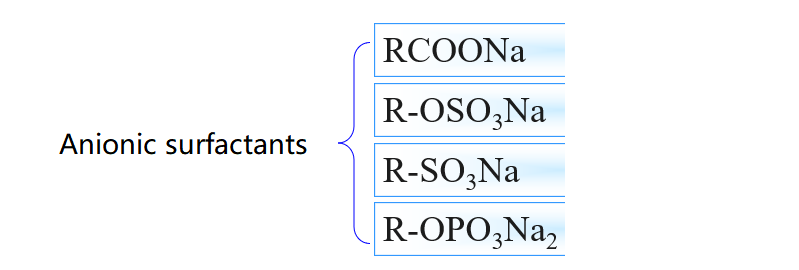
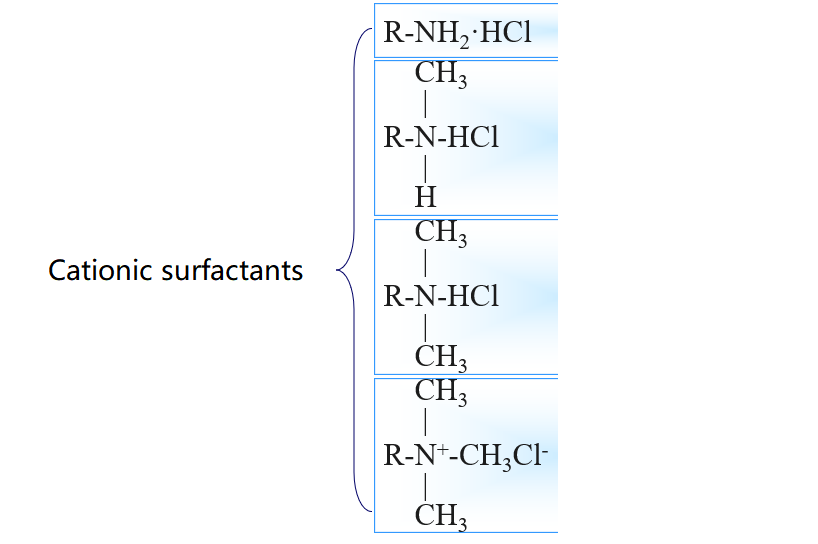
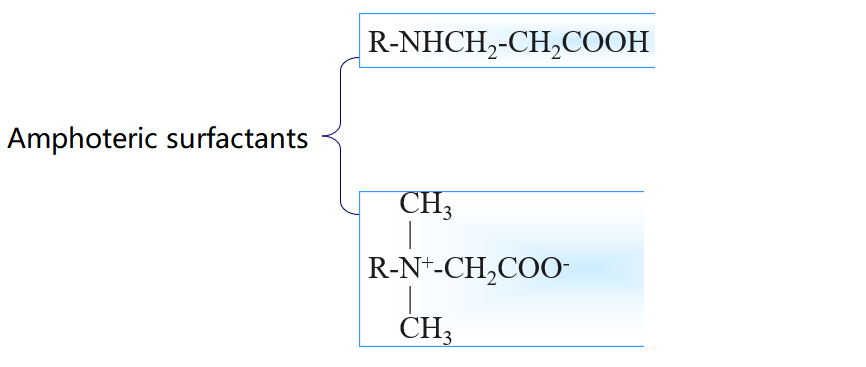
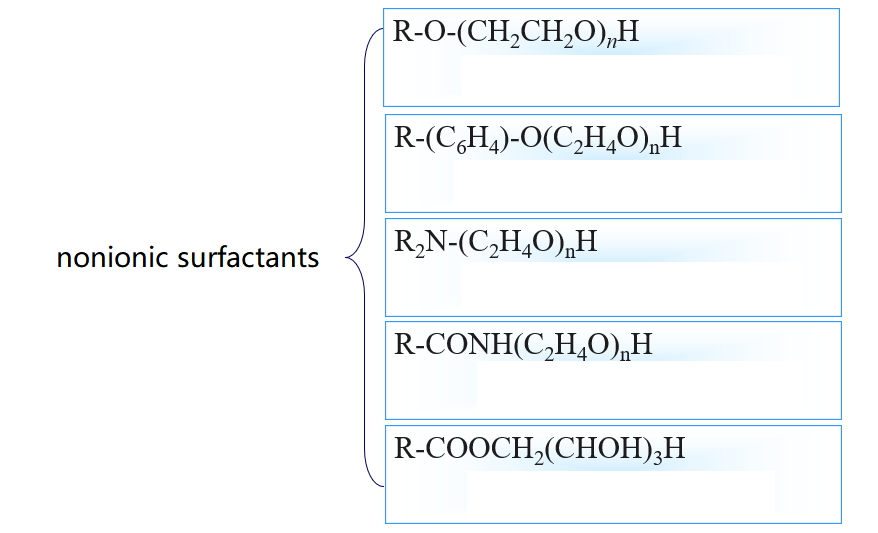
4.Surfactant Efficiency
The concentration of surfactant required to reduce the surface tension of water to a certain value. Obviously, the lower the required concentration, the better the performance of the surfactant.
5.Surfactant Capacity
The minimum value to which a surfactant can reduce the surface tension of water. Obviously, the lower the surface tension of water, the more effective the surfactant is. The ability of a surfactant is also called its effective value.
6.The Impact of Surfactant Structure on Its Efficiency and Capacity
The efficiency and capacity of surfactants are often numerically inversely proportional.
Increasing the chain length of the hydrophobic group increases the efficiency of the surfactant, but the capacity may decrease.
Increasing the number of hydrophobic groups with branched chains or increased unsaturation reduces the efficiency, but increases the capacity.
7.Micelles and Critical Micelle Concentration
As the concentration of surfactant in water increases, the surfactant molecules aggregate on the surface to form a tightly arranged monolayer, and the excess molecules in the bulk phase also approach each other in groups of three or two with hydrophobic groups, gathering together to form micelles.
The minimum concentration at which micelles can form is called the critical micelle concentration (CMC).
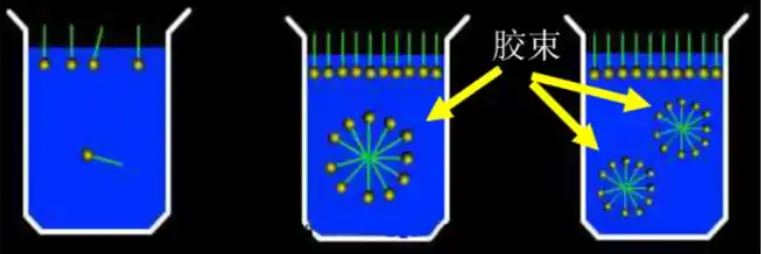
Depending on the hydrophilic groups and their concentrations, the micelles formed can take on various shapes such as rods, layers or spheres.
The properties of the solution forming micelles deviate from the ideal properties, and a turning point appears on the curve plotting surface tension against concentration. Continuing to increase the concentration of the surfactant, the surface tension no longer decreases, while the number and size of micelles in the bulk phase continue to increase.
8.HLB Value of Surfactants
Griffin proposed using the HLB (hydrophile-lipophile balance) value to indicate the hydrophilicity of a surfactant.

For example, paraffin wax has no hydrophilic groups, so its HLB value is 0.
Polyethylene glycol, on the other hand, has only hydrophilic groups and an HLB value of 20.
You can choose the appropriate surfactant based on its HLB value.
For example, an HLB value between 2 and 6 is used as an oil-in-water emulsifier; an HLB value between 8 and 10 is used as a wetting agent; and an HLB value between 12 and 18 is used as an oil-in-water emulsifier.
9.Solubility of surfactants in water
The more hydrophilic a surfactant is, the greater its solubility in water, while the more lipophilic it is, the more soluble it is in oil. Therefore, the hydrophilicity and lipophilicity of a surfactant can also be measured by solubility or properties related to solubility.
(1) The solubility of ionic surfactants increases with increasing temperature. When a certain temperature is reached, the solubility increases suddenly and rapidly. This transition temperature is called the Kraff point.
The longer the hydrocarbon chain of the homologue, the higher its Kraff point. Therefore, the Kraff point can measure the hydrophilicity and lipophilicity of ionic surfactants.
(2) The hydrophilic group of nonionic surfactants is mainly polyoxyethylene. Increasing the temperature will destroy the bond between the polyethylene group and water, causing the solubility to decrease and even precipitation. Therefore, when heated, the solution will become turbid. The lowest temperature at which turbidity occurs is called the cloud point.
The fewer ethylene oxide molecules there are, the more hydrophilic it is and the higher its cloud point. Conversely, the more lipophilic it is, the lower its cloud point. The cloud point can be used to measure the hydrophilicity and lipophilicity of nonionic surfactants.
10.Some important functions and applications of surfactants
(1) Wetting effect: Surfactants can reduce the surface tension of liquids and change the size of the contact angle, thereby achieving the desired purpose.
(2) Foaming effect: A bubble is a spherical object formed by a thin film of liquid surrounding a gas. Some surfactants and water can form a film of a certain strength, surrounding the air to form foam. This is used in flotation, foam fire extinguishing, and washing and decontamination. This type of surfactant is called a foaming agent.
The main functions of foaming agents are:
(a) reducing surface tension;
(b) making the foam film firm, with a certain degree of mechanical strength and elasticity;
(c) ensuring that the foam has an appropriate surface viscosity.
(3) Solubilization
Non-polar organic compounds such as benzene have very low solubility in water. After adding surfactants such as sodium oleate, the solubility of benzene in water is greatly increased. This is called solubilization.
Note: Solubilization is different from the common concept of dissolution. The solubilized benzene is not evenly dispersed in water, but is dispersed in micelles formed by oleate molecules.
(4) Emulsification: Simple emulsions are usually divided into two categories. The organic matter that is insoluble in water is usually called oil, the discontinuous phase in the form of liquid droplets is called the internal phase, and the continuous liquid phase is called the external phase.
a. Oil-in-water emulsions are represented by O/W.
The internal phase is oil, and the external phase is water. This type of emulsion can be diluted with water, such as milk.
b. Water-in-oil emulsions are represented by W/O.
The internal phase is water, and the external phase is oil, such as crude oil gushing out of an oil well.
(5) Detergents
The decontamination process involves immersing a solid (s) containing dirt (denoted by D) in water (w). Under the action of detergents, the work of adhesion between the dirt and the solid surface is reduced, causing the dirt to fall off and achieving the purpose of decontamination.
A good detergent must have:
(a) good wetting properties;
(b) the ability to effectively reduce the interfacial tension between the cleaned solid and water, and between the dirt and water, thereby reducing the work of adhesion;
(c) a certain foaming or solubilizing effect;
(d) the ability to form a protective film on the clean solid surface to prevent the re-deposition of dirt.
In addition, surfactants are also used in many industrial fields such as textile industry, metal industry, coating, paint, pigment industry, plastic resin industry, paper industry, leather industry, oil extraction, building materials industry, mining industry, energy industry, etc. In short, surfactants play an important role in various industries due to their unique molecular structure and multiple functions.